Batteries/Energy Storage
Nissan's battery assembly line.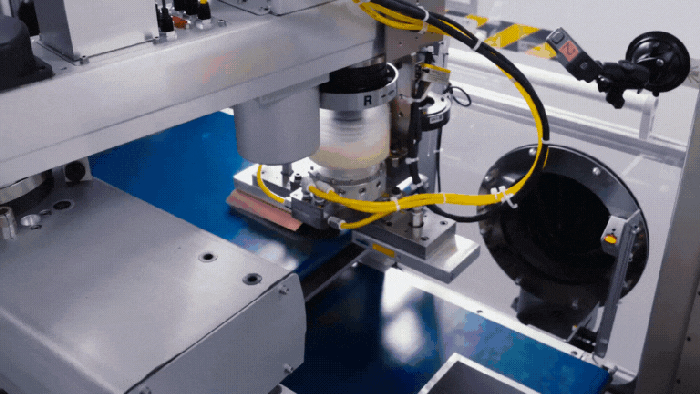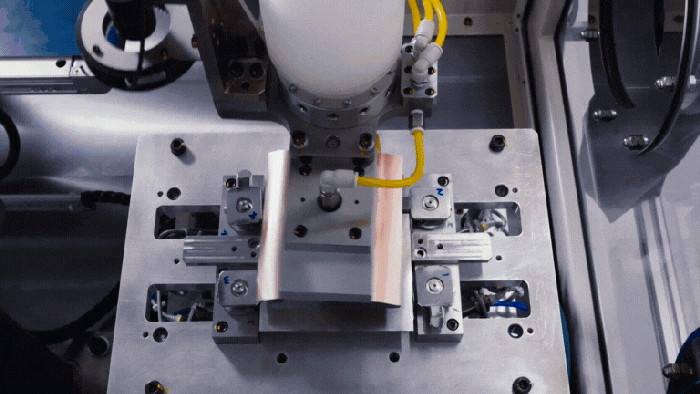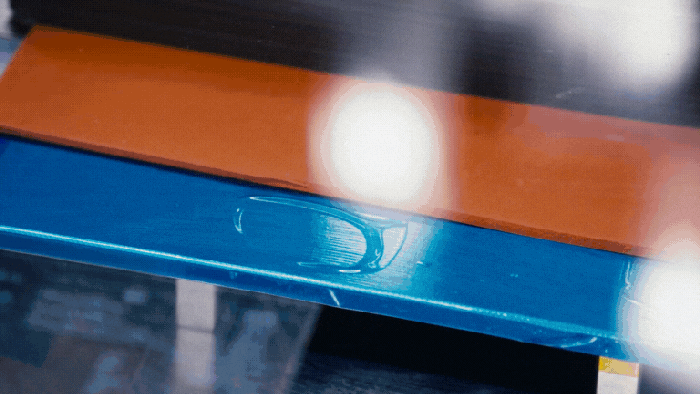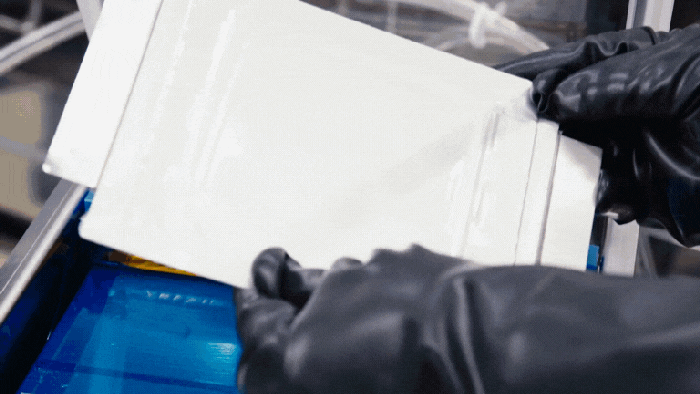
Automotive Engineering
Nissan Solid-State Battery Plant Nears Production ReadinessNissan Solid-State Battery Plant Nears Production Readiness
Nissan is poised to put solid-state EV batteries into production by 2028.
Sign up for the Design News Daily newsletter.








.gif?width=300&auto=webp&quality=80&disable=upscale)


.gif?width=300&auto=webp&quality=80&disable=upscale)
.jpg?width=300&auto=webp&quality=80&disable=upscale)



















![Keysight Battery Test Lab[22] copy.jpg Keysight Battery Test Lab[22] copy.jpg](https://eu-images.contentstack.com/v3/assets/blt0bbd1b20253587c0/blt7dd171be607c745b/651429716a746399bb54f0d6/Keysight_20Battery_20Test_20Lab22_20copy.jpg?width=300&auto=webp&quality=80&disable=upscale)






.jpg?width=300&auto=webp&quality=80&disable=upscale)
