OpenPLC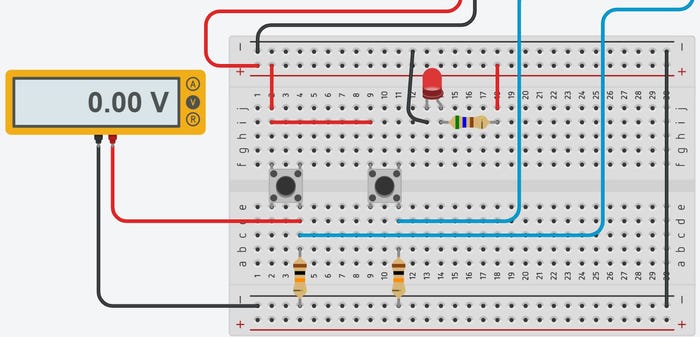
Components & Subsystems
What Is OpenPLC?What Is OpenPLC?
This project for building an Arduino Uno or compatible PLC illustrates OpenPLC’s effective and low-cost tools for automation development.
Sign up for the Design News Daily newsletter.

This project for building an Arduino Uno or compatible PLC illustrates OpenPLC’s effective and low-cost tools for automation development.