GE's Biopharma Plant Solution: Can't Export Drugs? Export the Factory
December 22, 2015
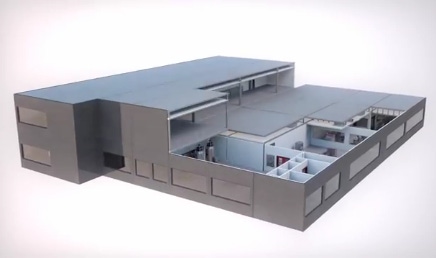
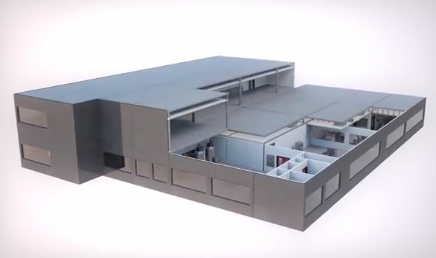
Last month, GE made a curious shipment: Instead of a machine or a part, it shipped an entire factory from Germany to China. The prebuilt biopharmaceutical plant was built in Stuttgart and then disassembled and packed in 62 containers; sailed down the Rhine, across oceans, and up the Yangtze River, where the buyer of the factory, a startup company called JHL Biotech Inc., had prepared ground and utilities for it. JHL plans to tap into the lucrative and growing market for biopharmaceuticals in China, but why would it purchase a factory built in Europe?
Olivier Loeillot, general manager of commercialization in Asia for GE Healthcare Life Sciences' BioProcess business, told Design News that the factory, called KUBio, is a way to be sure standardized quality is attained so that the customer can quickly earn Good Manufacturing Practice (GMP) and other regulatory approvals and be up and running. KUBio's pre-installed equipment is fully qualified up to operational qualification (OQ), which fast-tracks the factory to production-ready status once the modules are assembled and eliminates delays associated with traditional plant construction.
"As well as being pre-designed to meet CGMP (Current Good Manufacturing Practice) compliancy, the modular design allows any other necessary validation processes to begin before site completion," Loeillot told us. "Streamlined approval and validation means a shorter time to the start of production."
It's also a way of "de-risking" for investors, which is an important factor for startup companies looking to move themselves forward. But what makes it an ideal approach is that the Chinese government requires that all drugs sold in China be manufactured in the country and approved by the State Food and Drug Administration (SFDA).
The modules are designed around GE's FlexFactory platform to minimize the number of joints and amount of pipework and therefore the footprint of the building.
"What makes KUBio innovative is the standardized nature of the layout, making the facility reproducible in any location, to a repeatable high quality, and be CGMP compliant," Loeillot said. "Additionally, KUBio supports tech transfer and scale-up, so it's a good solution for biomanufacturing capacity for young companies that may not have the headcount to manage a multifaceted project by themselves."
READ MORE ARTICLES BY TRACEY SCHELMETIC ON DESIGN NEWS:
Because it's a biopharmaceutical facility, the prebuilt factory comes with cleanrooms along with pipework, gas fittings, heat, ventilation systems, and even restrooms. Interestingly, the factory is built to accommodate single-use equipment rather than reusable stainless-steel equipment that must undergo labor-intensive rigorous decontamination processes between uses. Processing with single-use equipment reduces the number of unit operations compared with traditional stainless-steel equipment, which in turn reduces the overall plant footprint even further. In addition, it enables the facility to produce multiple drugs with no risk of cross-contamination.
Currently, KUBio is intended for the manufacture of mammalian cell-based biopharmaceuticals -- specifically monoclonal antibodies. The FlexFactory platform, however, can be applied to the manufacture of other types of biologics, such as vaccines, which ideally should be manufactured close to the point of use.
"Ultimately, the modular, standardized approach of KUBio means that much-needed world-class pharmaceuticals can be produced anywhere in the world, close to the point of need," Loeillot said.
Tracey Schelmetic graduated from Fairfield University in Fairfield, Conn. and began her long career as a technology and science writer and editor at Appleton & Lange, the now-defunct medical publishing arm of Simon & Schuster. Later, as the editorial director of telecom trade journal Customer Interaction Solutions (today Customer magazine) she became a well-recognized voice in the contact center industry. Today, she is a freelance writer specializing in manufacturing and technology, telecommunications, and enterprise software.
Like reading Design News? Then have our content delivered to your inbox every day by registering with DesignNews.com and signing up for Design News Daily plus our other e-newsletters. Register here!

Design engineers and professionals, the West Coast's most important design, innovation, and manufacturing event, Pacific Design & Manufacturing, is taking place in Anaheim, Feb. 9-11, 2016. A Design News event, Pacific Design & Manufacturing is your chance to meet qualified suppliers, get hands-on access to the latest technologies, be informed from a world-class conference program, and expand your network. (You might even meet a Design News editor.) Learn more about Pacific Design & Manufacturing here.
About the Author(s)
You May Also Like





