Medical Device Plastics Toughen Up for Harsh Hospital Environments
More plastics are coming into hospital equipment, as well as single-use, patient-friendly medical devices and wearables.
February 3, 2016
The demand for plastics that stand up to hospital disinfectants and plastics that help engineers design smaller, single-use, patient-friendly devices continue to be the major materials trends in medical device applications. OEMs also continue to replace metals with plastics in some medical equipment, and are designing more healthcare wearables.
Methods for dealing with hospital-acquired infections continues to be a main topic of interest among device and equipment OEMs, said Dane Waund, global market manager for healthcare for Solvay Specialty Polymers. It's important to distinguish between sanitized and sterilized instruments, and the materials that withstand those environments, to specify the right plastic for the right job.
[Visit Solvay Specialty Polymers at Booth 2144 at Pacific Design & Manufacturing, Feb. 9-11, at the Anaheim Convention Center.]
Specialty plastics are designed to undergo repeated sterilization. However, many equipment housings made from commodity plastics are now subject to sanitization, but those materials weren't designed for patient contact. "In the past, these didn't get decontaminated after every use," said Waund. "But now they are: hospital workers wipe down surfaces daily and spray them with disinfectants. So there's definitely a trend toward replacing those commodity plastics with engineering plastics, or even with specialty plastics because some people just don't want the risk."
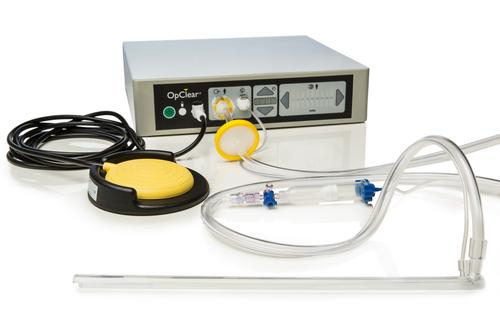
A laparoscope lens cleaner developed by Cipher Surgical includes OpClear's attachment, now made of Eastman Tritan copolyester. This material lets the attachment be securely clipped to a laparoscope without breaking, and provides toughness, clarity, flexibility, and resistance to sterilization processes.
(Source: Eastman Chemical Company/Cipher Surgical)
Waund said Solvay plans to announce soon some specific grades of Veradel PESU (polyethersulfone) for limited patient contact healthcare applications. This will be based on ISO 10993 biocompatibility testing, and reinforced via an FDA Master Access File for the Veradel products. The decision to support PESU for healthcare was prompted by customer demand. Veradel PESU addresses performance gaps between PSU (polysulfone), polyethersulfone (PESU), and PPSU (polyphenylsulfone), which Solvay already supports, in areas such as rigidity, flow, and sterilization system compatibility.
One of the biggest changes Eastman Chemical Company has seen in demand is more customers looking for polymers that stand up to the disinfectants hospitals are using now, said Cynthia Lewis, the company's market insight and strategy manager. Recent regulation changes reduce Medicare and Medicaid reimbursements to hospitals with higher-than-specified hospital acquired infections. "So they're using a lot of bleach and alcohol very aggressively throughout the hospital," she said. "Infection rates are dropping. But many polymers used in hospitals are breaking down: the outer casings on monitoring equipment, for example, are cracking and even crumbling." Even with plastics that have antimicrobial properties, it's difficult to tell when that material is no longer effective, so the solution is to use a lot of disinfectant everywhere.
Eastman's Tritan copolyester material is designed to provide the toughness and chemical resistance needed for making medical devices safe and reliable, said Lewis. As equipment becomes more portable it also becomes more wireless-enabled, so much of it runs on batteries. Since there are fewer AC plugs, it no longer requires the highest level of UL ratings. "Our Tritan MXF 121 is formulated to perform well in device housings, and it also retains a good surface quality after being wiped down with lots of disinfectant," she said. Eastman has other copolyesters for use in various types of medical equipment, including disposable devices, said the company's medical devices market development manager, Ellen Turner. She also cited the growing concern that materials be BPA-free and halogen-free, as Tritan is. "Device designers are saying more and more, as much as it costs and as long as it takes to design and launch a medical device, let's avoid chemicals of concern."
[Visit Eastman Chemical Co. at Booth 2407 at Pacific Design & Manufacturing, Feb. 9-11, at the Anaheim Convention Center.]

As the healthcare industry moves toward greater outpatient care and home administration of medication, manufacturers of drug delivery devices such as insulin pens seek easy-to use, compact, and aesthetically pleasing designs. SABIC's CYCOLOY HCX1640 resin helps device OEMs design robust, light, attractive drug delivery devices that meet standard healthcare regulations.
(Source: SABIC)
The environmental stress cracking caused by repeated wipedown of medical equipment with increasingly aggressive disinfectants affects medical devices as well as structural applications such as hospital bed components, and can be related to many factors, said SABIC Innovative Plastics' director of health care, Cathleen Hess. "They include polymer morphology, chemical concentration, and residual stress in molded components." The company's engineered thermoplastics, including healthcare grades of VALOX, XENOY, and ULTEM resins, were developed to help medical device manufacturers meet chemical resistance requirements.
Another major trend SABIC is seeing is pressure to reduce medical device system costs. This can be achieved in part with metal replacement. Using stronger, stiffer polymers and composites to replace metals in healthcare devices such as endoscopes enables part consolidation, design flexibility, manufacturing efficiencies, and weight reduction. SABIC is working with customers to replace metal with other material technologies, including high-modulus carbon fiber-reinforced thermoplastics that provide high strength and stiffness, chemical compatibility, and the mechanical stability required for repeated, harsh sterilization environments. Metal replacement is also occurring when multi-use sterilizable devices, often made with metals, are replaced with more single-use, disposable devices made with plastics.
About the Author(s)
You May Also Like



