First 3D-Printed Titanium Cranial Implant To Get FDA Approval
The Food & Drug Administration has approved a 3D-printed, titanium, cranial/craniofacial patient-specific plate implant for use in the US. The implant is 3D printed using Arcam's electron beam melting (EBM) process.
February 1, 2016
The FDA (Food & Drug Administration) has approved a 3D-printed, titanium, cranial/craniofacial patient-specific plate implant for use in the US. The implant was designed by Brazil-based BioArchitects, and is 3D printed using Sweden-based Arcam's electron beam melting (EBM) process.
Designed for repairing defects in the non-loadbearing bones of the head and face, each custom-designed plate is permanently attached to the skull and/or face with self-tapping titanium screws, Mark Ulrich, CEO of BioArchitects USA, told Design News. Previously, titanium cranial/craniofacial plate implants sold in the US have been machined or foundry-based. The new 3D-printed implant design takes advantage of the light weight and tensile strength of a biocompatible titanium alloy.
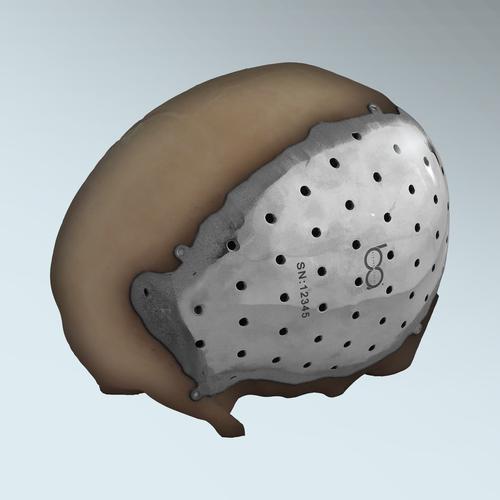
The first patient-specific, 3D-printed, titanium cranial implant in the US has been approved by the FDA, designed by BioArchitects and produced using Arcam's electron beam melting (EBM) process. 3D printing integrates the attachment heads into the plate, improving tensile strength over that of separate attachment brackets used for conventionally made implants.
(Source: BioArchitects)
"Unlike others in the market, this is the only implant that has, as part of its architecture, attachment heads that are an integral part of the plate itself, without needing separate attachment brackets for the plates," said Ulrich. "The counter-sunk, hole-drilled tabs' location and positioning in each case are based on collaboration between the surgeon and the biomedical engineer. These only require off-the-shelf, standard titanium screws to screw the plate into the native bone. Most others require sourcing additional attachment brackets to adhere the implant to the bony surface."
As part of the process for acquiring the FDA's 510(k) clearance, BioArchitects was asked to do mechanical testing on the plate. "That included the weakest portion, which in this case is the attachment tabs," said Ulrich. "In laboratory testing, we found that their tensile strength is three to four times as great as the tensile strength of the commercially available titanium attachment brackets."
[Learn more 3D printing trends and developments at Pacific Design & Manufacturing, Feb. 9-11, at the Anaheim Convention Center.]
Such custom implants are designed to repair bony defects that result from congenital abnormalities, trauma, or disease. They are created by importing a CT scan or MRI of the affected area into a specialized design program to make a template of the repair, which becomes a model for fabricating the 3D-printed plate. Multiple finishes are possible: mirror, partially polished, unpolished margin and edge, or unpolished surface, said Ulrich, depending on how much osseointegration is needed for the particular repair.
READ MORE ARTICLES ON 3D PRINTING IN MEDICAL APPLICATIONS:
Arcam has supplied orthopedic implants for over a decade made with its EBM systems. The company recently expanded operations in the US by opening a new sales and service office in Woburn, Mass. called Arcam CAD to Metal. Its EBM process is also well-known in aerospace manufacturing.
Ann R. Thryft is senior technical editor, materials & assembly, for Design News. She's been writing about manufacturing- and electronics-related technologies for 27 years, covering manufacturing materials & processes, alternative energy, and robotics. In the past, she's also written about machine vision and all kinds of communications.

About the Author(s)
You May Also Like



