Could This Discovery Make More Polymers Recyclable?
Researchers have developed a technique to introduce breakable bonds into thermoset polymers so they might be better suited to recycling processes.
February 9, 2024
At a Glance
- Thermoset polymers burn rather than melt when heated, making them difficult to recycle
- Researchers experiment with breakable bonds in polymer chains that may allow materials to retain properties when reformed
Scientists in the UK have developed a technique that could make a whole range of polymers far easier to recycle than they currently are, paving the way for more sustainability in the use of plastic materials.
Researchers at University of Bath and University of Surrey developed a way to introduce degradable bonds into thermoset polymers to make them more easily recyclable. From this technique they hope to develop a roadmap for the industry to understand better plastic materials to promote recycling of more of them, they said. Currently only a small percentage of the plastic materials we use can be recycled.
“Thermosets are used widely in the commercial sector, in materials like resins and adhesives," explained Maciek Kopeć, a professor and researcher in the University of Bath’s Department of Chemistry and one of the leaders of the research. “Being able to make bonds reversible in these materials will increase their applications as well as making them more recyclable.”
In addition to recycling, other applications for the work may include using cross-linked polymers as vehicles for controlled drug delivery systems, he said.
Understanding Polymers
Plastic materials are composed of long chains of molecules called polymers but behave differently when heated. Typically thermoset polymers—often used in composite materials such as rubbers, gels, and adhesives—burn rather than melt when heated, making them difficult to recycle.
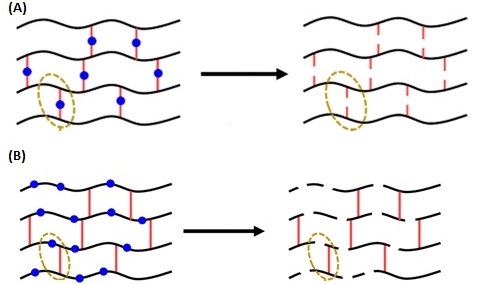
That's partly because of their structure, which is composed of polymer chains that are cross-linked to form a network, making them incredibly strong and flexible. However, the cross-links are what contribute to the material burning rather than melting.
Thermoplastics, on the other hand, are the ones typically recycled today. They can be heated to high temperatures, poured into a mold, and then cooled to make the desired shape. This means that they also can be melted and reformed into other shapes when they are recycled, even though they are more prone to break when stretched or stressed.
Model for the Future
To try to change the properties of the materials to promote recycling, the researchers synthesized a series of polymer gels with breakable bonds incorporated into different parts of the thermoset material's structure. They then tested whether the properties changed after the gel was degraded and reformed to find the most viable bonds to spur change in the material.
What they found is that while all the gels could be degraded to some extent, gels with breakable bonds in the polymer chains retained their properties much better when reformed. This compared with the polymers that the cross-linked bonds broke down, they said.
The researchers published a paper on their work in the journal, Polymer Chemistry. They envision that their model system also can be applied to other types of polymers, including adhesives, sealants, and elastomers.
They also plan to create a map so other scientists can find the best locations in the materials for breakable bonds as well as to help them understand better why some bonds break more easily than others. Other future plans include optimization of the system using other commercially used polymers, they said.
About the Author(s)
You May Also Like



