New Device May Boost Artificial Pancreas Efforts
MIT researchers built mechanical actuation into a soft robotic device, a design that could be a game changer for artificial pancreas systems.
August 9, 2022
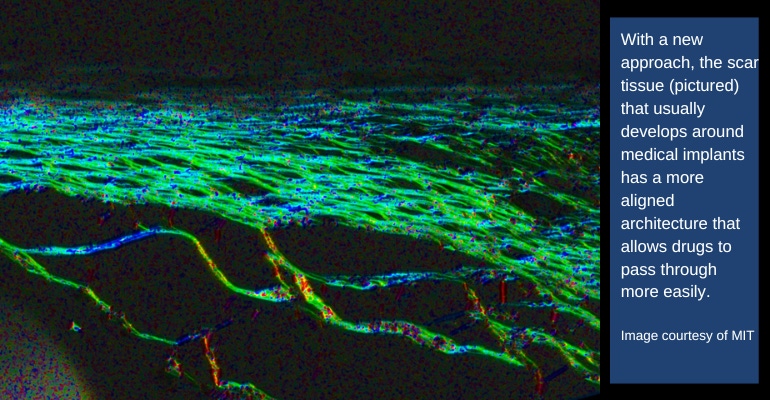
A team of MIT engineers and collaborators may have solved a major hurdle where implantable medical devices are concerned, particularly devices designed to be used as an artificial pancreas in patients with diabetes.
What tends to happen after such a device is implanted is that immune system's foreign body response is triggered and scar tissue is formed around the implant, blocking the release of insulin or other drugs. Back in 2019, a team of researchers from MIT and Ireland published a study in Science Robotics that described how the use of mechanically actuated soft robotics could modify the body's response to implanted medical devices. Now, the team has taken the research a step further to show that the same approach could potentially improve drug delivery for diabetes patients and others.
The team built a two-chambered device made of polyurethane, a plastic that is commonly used in medical devices and has has similar elasticity to the extracellular matrix that surrounds tissues. One of the chambers acts as a drug reservoir, and the other acts as a soft, inflatable actuator. Using an external controller, the researchers can stimulate the actuator to inflate and deflate on a specific schedule. For this study, published in Nature Communications, they performed the actuation every 12 hours, for five minutes at a time.
According to the researchers, the mechanical actuation drives away immune cells called neutrophils, the cells that initiate the process that leads to the formation of scar tissue. When the researchers implanted these devices in mice, they found that it took much longer for scar tissue to develop around the devices. Scar tissue did eventually form, but its structure was unusual, they noted. Instead of the tangled collagen fibers that built up around static devices, collagen fibers surrounding actuated devices were more highly aligned, which the researchers believe may help drug molecules to pass through the tissue.
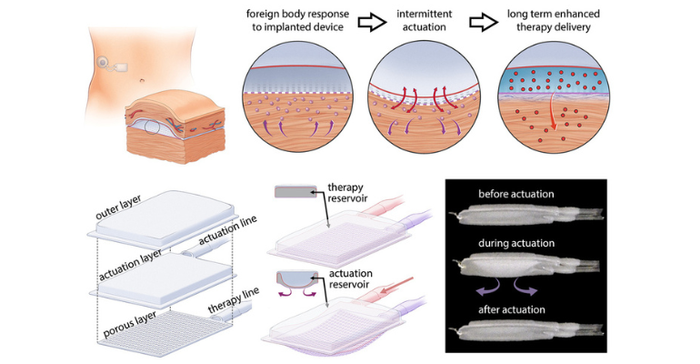
To demonstrate the potential usefulness of this device, the team showed that it could be used to deliver insulin in mice. The device is designed so that insulin can slowly seep out through pores in the drug reservoir or be released in a large burst controlled by the actuator.
The researchers evaluated the effectiveness of the insulin release by measuring subsequent changes in the mice’s blood glucose levels. They found that in mice with the actuated device, effective insulin delivery was maintained throughout the eight weeks of the study. However, in mice that did not receive actuation, delivery efficiency began to wane after only two weeks, and after eight weeks, almost no insulin was able to pass through the fibrous capsule.
The authors also created a human-sized version of the device, 120 millimeters by 80 millimeters, and showed that it could be successfully implanted in the abdomen of a human cadaver.
Among other possible applications, the researchers plan to see if they can use the device to deliver pancreatic islet cells that could act as an artificial pancreas to help treat diabetes, MIT News reported.
Co-authors of the study are Ellen Roche, associate professor of mechanical engineering at MIT, and a member of MIT’s Institute for Medical Engineering and Science; and Eimear Dolan, a former postdoc in Roche's lab who is now a faculty member at the National University of Ireland at Galway (NUI Galway). Lead authors of the paper are MIT postdocs William Whyte and Debkalpa Goswami and visiting scholar Sophie Wang. Garry Duffy, a professor of anatomy and regenerative therapies at NUI Galway, is also a key collaborator on the work.
Next steps: exploring artificial pancreas and other applications
Working with Jeffrey Millman, an associate professor of medicine and biomedical engineering at the Washington University School of Medicine in St. Louis, MO, the team plans to adapt the device for the delivery of stem-cell-derived pancreatic cells that would sense glucose levels and deliver insulin when glucose is too high. The researchers say that such an implantable device could eliminate the need for patients to constantly measure their glucose levels and inject insulin.
Among other possible applications, the researchers say they plan to see if they can use the device to deliver pancreatic islet cells that could act as an artificial pancreas to help treat diabetes.
The researchers have also explored potential applications outside of diabetes using this type of device, such as the delivery of immunotherapy to treat ovarian cancer, and delivering drugs to the heart to prevent heart failure in patients who have had heart attacks.
To read more about this research, click here.
The artificial pancreas dream and muddied messaging
In September 2013, headlines heralded FDA approval of the first artificial pancreas, prompting many patients with type 1 diabetes to revel in the realization of a long-awaited fantasy that has promised to revolutionize diabetes management. Unfortunately, Medtronic's MiniMed 530G wasn't exactly the dream device the diabetes community had in mind when they thought about the artificial pancreas dream. While the media’s misinterpretation of the verbiage was somewhat to blame, the muddy messaging really stemmed from FDA’s classification of threshold-suspend device systems as a type of artificial pancreas device system.
Frustrated with the misunderstanding and hype around the 530G, Leighann Calentine, author of the D-Mom Blog, which focuses on her experiences as the parent of a diabetic child, vented in a post a couple weeks after the device was approved. “The Medtronic 530G does one thing now that other existing products on the market do not do…It’s not a game changer,” she wrote. “Of course, it’s great that the first milestone on the way to an FDA-approved artificial pancreas has been reached. But, like I’ve said, it’s a first step. There’s no reason to throw a party.”
As MD+DI pointed out at the time, however, the MiniMed 530G was the beginning of a major paradigm shift in diabetes management, making the device a game changer even though it wasn't a fully automated artificial pancreas.
FDA includes three main categories of devices under the umbrella of artificial pancreas delivery systems. They differ in how the insulin pump acts on readings from the continuous glucose monitoring system. In addition to threshold-suspend device systems, FDA also considers insulin-only systems and bi-hormonal control systems to be a type of artificial pancreas system.
About the Author(s)
You May Also Like





