How to Build Better Medical Microelectronics
Could integrated computing power and microelectronic building blocks help you deliver next-gen implantable sensors, wearable devices, or portable instruments?
September 20, 2023
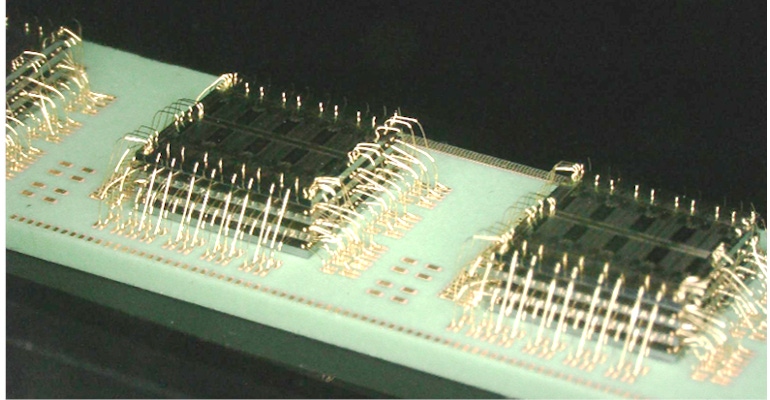
Advances in semiconductors, materials, power, and more are enabling electronics to miniaturize—including medical electronics. Design News asked Dave Fromm, Promex’s new chief operating officer, a few questions about engineering microelectronics for medical devices.
Fromm previously served as vice president of engineering for Promex, and he now leads operations, engineering, and business development teams from the company’s Silicon Valley facility in Santa Clara, CA. Promex offers microelectronic component assembly, process design, and packaging services for the medtech and biotech markets. It runs two full SMT lines; performs post-wafer processing such as including wafer dicing, grinding, die attach, and flip-chip; and offers wire bonding, encapsulation, and more.
Promex is exhibiting at BIOMEDevice Boston (Booth #347) September 20-21 and at MD&M Minneapolis (Booth #3426) October 10-11.
How are microelectronics being used in the medtech industry, and what are some potential future uses?
Fromm: Improved semiconductor fabrication technologies increasing computing power have impacted medtech in two ways. First, raw computing power has become more accessible for integration into medtech devices at the chip level—what was once the domain of specialized industries is now viable for integration into medical devices where the computer is a tool to enable other complex functionality. Second, microelectronics density has just made things smaller, consuming ever less power. This combination enables levels of integration that were only dreamed about a decade ago—be they tiny implantable devices with active sensors, wearable devices, or smaller portable instruments with high capability and simplified user workflows from automation. With the parts commercially available, the challenge now becomes how to integrate and assemble a manufacturable, reliable product.
Could microelectronics applications in other industries inspire new medical uses?
Fromm: Absolutely! This democratization of commercially available integrated computing power and microelectronic building blocks is creating a new frontier in medical devices. Product designers are now able to make a string of revolutionary products that address new issues as opposed to making evolutionary products that incrementally improve what is typically done.
What qualifies as microelectronics, and how micro could/should they get?
Fromm: We think of microelectronics as sub-devices with size scales described in microns (millionth of a meter; 100 microns is about the thickness of a human hair). These size scales require assembly tools/processes that control locations to a few microns and materials that enable one to actually put them together in three dimensions and enable field reliability.

Are reliability and longevity issues in medical microelectronics, and how can they be addressed?
Fromm: These are incredibly important issues. Ensuring continued quality through the device’s operational life is central to product design. Proper integration/miniaturization naturally improves reliability. At the first order, devices last longer with shorter path lengths within a device—the physical world just has less to interact with. However, new issues arise in microdevices. Most critical is properly managing the interfaces between different materials; care must be made in keeping interfaces clean during assembly, using proper materials to ensure reliable interfaces, and managing power within the device. Further, observing and understanding potential or observed reliability issues is much more challenging than in larger devices. Special tools often must be used to visualize defects or other issues throughout development, manufacturing, and upon release to the field. This is an art unto itself!
What are the latest advances in powering microelectronics?
Fromm: As these areas improve, power becomes the main driver of miniaturization. First, one needs to provide it; big pushes are for increased power density and lower-cost microbatteries or via inductive coupling to an external power source (where longer range and higher transfer efficiency are the key issues for improvement). Second, the devices need to consume less power.
What are the latest advances in chip design and functionality?
Fromm: Chips are no longer (basically) two-dimensional devices. Designers now wish to stack/array several devices within a single package to increase performance and integrate varied functionality nominally called chiplets. Microsensors are chip-based electromechanical devices.
Are there any advances in materials that could benefit microelectronics?
Fromm: Adhesives for assembly are perhaps the most critical development area for microelectronics integration. You may not think about it, but integration requires an incredible breadth of functionality from adhesives. Some examples are controlling viscosity so that the glue always goes where the design intends but never where it shouldn’t, creating adhesives with specific optical properties, creating adhesives with particular biocompatibility and/or chemical stabilities, and creating materials with desired electrical and/or thermal conductivity or mechanical properties for strength and reliability.
Are there any end-use or environmental challenges that microelectronics face, and how can they be addressed?
Fromm: Ship, store, and use conditions for microelectronics devices are changing tremendously as applications expand. This leads to new requirements for chemical stability and biocompatibility as well as temperature, humidity, and vibration/shock resistance.
How does Promex work with medtech designers on microelectronics? What are some common designer requests and how can you meet them?
Fromm: Promex works with medtech designers at all ends of the product development spectrum. We see some partners come in with a napkin sketch or simple design model for a concept and we work together on how to actually figure out how to build it, impacting design elements, material selection, and component selection. Throughout the process, we identify key risks. Other partners have a mature product in production and have specific problems they need help solving for manufacturing. And others are somewhere in between.
When designing and engineering a microelectronics medical device, are standard designs and materials typically used, or is there a lot of customization?
Fromm: The creativity of product designers and various issues with modern medical devices necessitates endless customization, especially as these devices getting ever smaller. However, the most successful designs leverage design elements and assembly processes that are well established, often in different markets. The trick is how to use something “old” in a new way.
What is needed to build better medtech that integrates microelectronics?
Fromm: More people coming into the field. Luckily, we’re seeing non-medtech young professionals, such as electrical engineers, have ideas for such emerging applications as neuromodulation, because they see an unmet need and have a proposed solution to a medical issue. We are seeing more and easier cross-pollination and cross-fertilization today than we have seen in the past between technologies, industries, and experts. Smart people and good ideas will continue to advance medtech that integrates microelectronics.
About the Author(s)
You May Also Like





