Design Verification and Design Validation: What's the Difference?
Although it’s easy for professionals to incorrectly interchange the terms, "design verification" and "design validation" have very different meanings.
September 11, 2015
Although design verification and design validation have very different meanings, it’s easy for professionals to incorrectly interchange the use of the terms. Here’s a refresher to denote the differences between design verification and design validation from a medical device perspective and show how each is properly utilized throughout the design engineering and development process.
Definition and Differences
Design verification is defined as, “confirmation by examination and provision of objective evidence that specified requirements have been fulfilled.” Design validation is, “establishing by objective evidence that device specifications conform with user needs and intended use(s)” (21 CFR 820.3). Simply put, verification confirms that the design output meets the design input requirements, while validation ensures that user needs are met by the medical device. For a simple visual relationship between them, see the following figure:
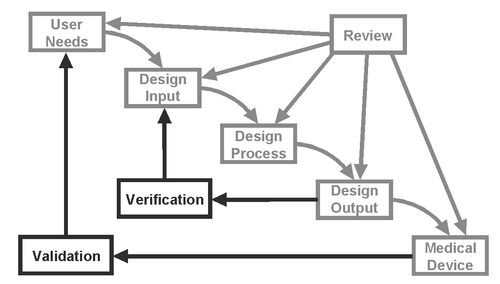
(Source: FDA CDRH 1997 Design Control Guidance for Medical Device Manufacturers)
To highlight the differences between the two, let’s say a user need is that the “device will be stable during use.” This user need could then generate the corresponding design inputs of “device will not tip over under 20 pounds of load” and “settling time will be less than 5 seconds after movement.” Tests could be created and performed on appropriate samples to characterize tip-over force and settling time in order to verify that the device meets each of these design inputs. To validate the device, on the other hand, engineers would use a completed production version (or equivalent) of the device to demonstrate that the user need of stability has been fulfilled during actual or simulated use.
READ MORE ARTICLES ON DESIGN VERIFICATION AND VALIDATION:
Verification
Verification can occur during different phases of design development. Some examples of early verification activities include:
• Comparisons (e.g., comparing standard human factors data to device features)
• Reviews (e.g., evaluating supplier component specifications to design inputs)
• Analysis, inspections, and measurements (e.g., evaluating parts for strength)
As the design matures, comprehensive verification test plans should be written to ensure that the design output meets all design inputs. These test plans should:
• Be pre-approved and show sufficiently detailed test procedures
• Specify the quantity and appropriate samples to be tested (e.g., how sample will be built, different configurations, etc)
• Establish pass/fail criteria before each test begins
• Be carried out with qualified equipment and objective personnel.
Note that previous test data from other projects may be used as long as it is adequate for the current application.
Validation
While formal validation activities should begin after design transfer to production specifications, design validation planning can start as soon as user needs and intended uses are specified. Typical validation activities include:
• Analysis of relevant scientific literature
• Comparisons to similar devices on the market
• Usability testing (e.g., effectiveness of user interfaces and labeling)
• Simulated use of the device by users
• Clinical trials (e.g., actual use of device per IDE regulations).
When performing design validation, it is important to specify:
• Environment and operating conditions
• Types of users (abilities, skills, and knowledge)
• Type of use (actual or simulated)
• Use of initial production units (or their equivalent).
As with verification, always establish acceptance criteria (subjective or objective) before tests begin. Finally, remember that final design validation should follow successful final design verification; not come before it.
Although design verification and design validation share common attributes, they have many distinct differences. In summary, the way to distinguish them is verification answers the question, “Did we build the device right to meet the requirements?” and validation answers the question, “Did we build the right device for the user?”

We’re heading to Philly and Houston! Design & Manufacturing Philadelphia will take place Oct. 7-8, while Design & Manufacturing Texas will be in Houston Oct. 13-14. Get up close with the latest design and manufacturing technologies, meet qualified suppliers for your applications, and expand your network. Learn from experts at educational conferences and specialty events. Register today for our premier industry showcases in Philadelphia and Texas!
Greg Jung has more than 25 years of experience designing medical equipment and electro-mechanical products for a wide variety of industries. He also served in various project management roles and has led global, cross-functional development teams for a wide variety of programs. During this time, he developed several award-winning and patented product designs. Greg holds bachelor and master of science degrees in mechanical engineering from the Georgia Institute of Technology.
You May Also Like



