Design Considerations for Medical Device Navigation Systems That Enable Minimally Invasive Surgeries
Consider these design options for reimagining surgical devices for minimally invasive surgeries.
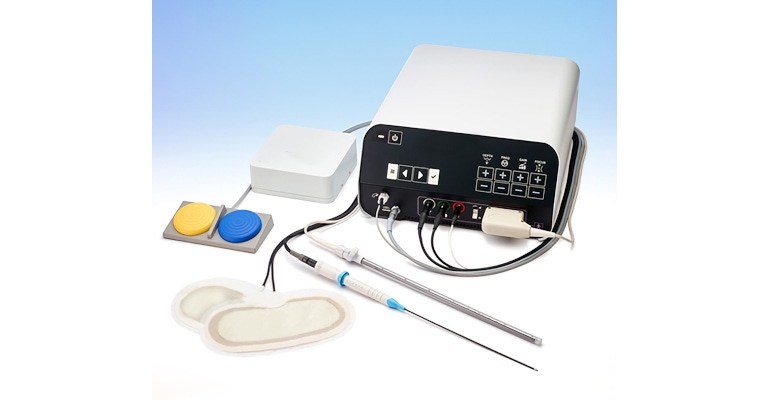
Since minimally invasive surgery (MIS) was pioneered in the ‘80s, it has become the standard of care for many procedures today. One study (Bingmer K, 2020. Feb; 34[2]) purports to show a 462 percent increase in cases using minimally invasive techniques between 2000 and 2018. These procedures not only alter the standard of care in intervention, but they also alter the patient journey through recovery, allowing patients to convalesce at home.
As demand for newer, faster, and safer procedures has steadily increased, so, too, have the navigation and optics-based options to facilitate them. The increase in available technology and methods to aid surgeons in performing minimally invasive procedures presents an opportunity for surgical device designers to re-imagine their portfolios to better aid perioperative navigation. Here are some design challenges frequently encountered and some tips on addressing them.
EM Nav and “Retrofitting” Existing Surgical Instruments
A surgical device company’s portfolio will already consist of dozens of instruments that have been tried and tested with surgical key opinion leaders to hold the strictest tolerances in performing their specific tasks. In many cases, the existing tool kits form the basis of surgical methods that are themselves the standard of care. Adding additional navigational capabilities presents an opportunity to offer a dramatic increase in value and functionality in a proven system and method.
One of the most straightforward ways to bring an existing surgical instrument portfolio forward to enable MIS is by adding electromagnetic surgical navigation (EM Nav) to it. This not only increases the value of the device, but it also extends the lifecycle of the instrument by ensuring that it keeps pace with accelerating surgical trends. Incumbent companies should evaluate their current portfolio for opportunities and either acquire or contract the right skillset to determine the best path forward: either an existing platform, or a bespoke system, designed to complement their toolkit. Either way, adding navigation capability will ensure physicians and patients are best served by their products.
Additive Components
EM Nav can offer additive value. By adding EM Nav components to an existing OEM device, a company can bring MIS to an existing, proven surgical method, without having to re-invent the instruments themselves or alter the primary mode of intervention.
Further, in an EM Nav system, multiple devices can be tracked simultaneously, without the need to make significant additions to surgical workflow (as when fluoroscopy is added to aid visualization).
An EM Nav system consists of a sensor, embedded into an OEM device, that serves as the localization point for the device in space; a field generator (usually placed under the patient) that creates the EM field that establishes the measurement volume; a sensor interface unit (SIU) and a system control unit (SCU) to detect the sensor’s signal and convert it to position and orientation information; and a display to allow the surgeon to see the position of the instrument in three dimensions without opening the patient.
For existing surgical instruments, the first design challenge is determining how to affix the sensor to the instrument itself. The additional components need to:
Isolate the position of the tip as accurately as possible—this often means placing a new component onto the device nearest the distal end.
Connect to the SIU/SCU, located usually outside of the sterile field.
Be able to consistently activate in the EM field, regardless of orientation in space.
Be biocompatible and otherwise not interfere with the procedure itself.
Each of these requirements has their own specific design challenges associated with them; however, they can be summarized as adding physical components to an existing platform that, at the same time, adds new functionality and does not detract from the existing, proven function of the device. As device engineers can attest, even simple, minor design changes can ripple through the device, into manufacturing, causing compounding headaches, extending timelines, and increasing budgets.
The Make vs. Buy Decision
With few exceptions, the trends toward MIS will exert pressure on the surgical instrument market to ensure that their devices enable these intervention methods. Companies looking at their instrument portfolio should consider how to enable MIS within it, and thus they will be confronted with an overall “make” vs. “buy” decision more complex than perhaps is typical.
Embarking on a program to add EM Nav to an existing toolkit will force the engineering department to look at their in-house talent to determine whether they have the availability as well as the correct skillsets at their disposal to develop such a system. Project managers may also, during the preplanning phase, consider in-house manufacturing capabilities to determine whether a retrofitted device could be made in-house or whether the company will need a commercialization partner.
Program leaders who conduct this analysis and find concerning gaps may be tempted to look for a partner with an existing platform that they claim is “ready-to-integrate.” The authors would caution deciders to bear in mind that protecting the integrity of the original device is paramount to physician adoption, and no existing system will be without design integration hurdles. Furthermore, given the rate of change in the industry, companies would do well to stay as flexible as possible, so they minimize the impact of software or hardware upgrades from a third party.
It is often better to bring in experts as early as possible to evaluate the program, educate on available solutions, and help write the requirements for the specific application. One might refer to this, within the context of a phase-gate process, as a “Phase 0.” Then, program leaders and their partners can make a much more informed decision about the process moving forward and what sort of compromises will need to be made and develop a comprehensive plan for them.
New Surgical Optical Systems
MIS comprises all minimally invasive surgeries, including those where direct visualization is paramount to the success of the procedure. Companies wanting to add direct visualization to their instrument portfolio will need to develop a system that is self-contained as well as specifically compatible with the tools and methods already established and validated.
Given the trends in the specific intervention that a company is serving, physicians may be best served by companies developing a complete optical system (or an optical system compatible with existing surgical visualization platforms) of their own. While starting a new product development program with a veritable “blank page” is often desirable, for companies new to surgical optics, there are some new pain points to bear in mind.
What Got You Here Won’t Get You There
Unlike developing a custom proof-of-concept EM Nav system, which often requires buying individual components and building subassemblies, there are options for engineers to build a prototype surgical optical system using existing, commercially available sub-assemblies. While this can speed some early-stage proof-of-concept work to demonstrate utility and value, these systems are almost never able to scale during development and will need most if not all their subsystems redesigned to be compatible with the application’s requirements.
Given how phase-gate programs are run, it can set a false sense of progress when Phase 1 yields a functioning prototype that needs to be fully redesigned in Phase 2. It can cause program leaders and sponsors to panic, reaching out to potential partners who are fragmented and forced to work in a confined requirement space to not derail the program.
Looking ahead to verification, validation, and commercialization, those subsystems used for prototyping will have individual components that are often very challenging to source. If you have predicated your design on something with exceptionally long lead times, or if a component is locked into an assembly and teams are hunting for base components, this can also significantly impact time to market, cost of goods, and simply being able to build enough devices to support testing.
Start From Where You Are
If direct visualization is the way to bring your firm’s surgical portfolio into the MIS space, it is best to begin at the beginning. As with EM Nav, early-stage work should be focused on proper system integration and have the entire program and product life cycles in mind from the start, as the program is taking shape and being dimensioned (for time, cost, risk, market value, etc.). Scalability in commercialization is paramount, and it is very common for firms engaged in these programs to struggle downstream in getting optical components sourced, assembled, and aligned within the strict tolerances that optical systems require. It is also challenging to achieve the numbers needed for validation.
The opportunity presented by having a bespoke optical MIS system in the portfolio cannot be overstated, but neither can the complexity and risk. Incumbent firms are well-advised to consider bringing in a design-development partner as early as possible, if for no other reason than to ensure that an experienced voice is available to discuss the potential pitfalls throughout. As with a new EM Nav system, adding a “Phase 0” before the program moves to planning guards against downstream risks derailing the program late in the timeline.
Surgical Guidance Is the Trend
The trend toward minimally invasive surgeries isn’t slowing, and now, as payers have discovered that at-home convalescence represents a significant cost savings, the drive to send patients home sooner will only create more opportunities for companies to service this market.
Incumbent surgical instrument firms already work to enable validated surgical methods and should therefore be at the forefront of developing this new generation of devices. Whether through retrofitting existing OEM instruments, or adding new optical instruments to the portfolio, the risks are clear, but careful pre-planning with established experts can help save time, money, and headaches.
About the Author(s)
You May Also Like



