PEEK Cranial Implant Debuts at MD&M
February 7, 2011
Significantadvances in implant technology will be among the highlights of the MedicalDesign & Manufacturing Showin Anaheim, CA Feb. 8-10.
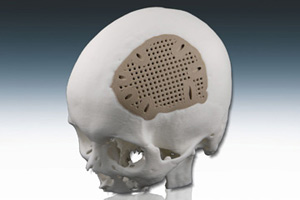
In one ofthe leading examples, EOS will be showingthe first PEEK craniofacial test implants to be produced with laser-sintering.
PEEK Cranial Implant Debuts at MD&M |
The testimplants were fabricated in Germany using the EOSINT P 800 system, which is thefirst laser-sintering system worldwide operating at up to 385C for processinghigh-performance polymers.
According toEOS, high-temperature, biocompatible PEEK (polyaryletherketone) material is increasinglybeing used as an alternative to titanium for craniofacial implants (producedfrom CT-scan geometry) for patients with head injuries or congenitaldeformities.
"Conventionalmanufacturing technology can't produce patient-customized craniofacialimplants, either titanium or PEEK, as economically or in as short a time spanas laser-sintering," says Joerg Lenz, collaborative projects coordinator forEOS with a European Union-funded project called Custom-IMD. "What's more, EOS technologyhas enabled us to develop a specific geometry that can only be realized usingadditive manufacturing. This new design incorporates a mesh scaffold thatpromotes improved bone growth and optimizes infiltration with ahydroxyapatite-filled, bio-absorbable polymer."
The final test implant is filled with bio-absorbable SupraB/hydroxyapatitecompound. Hydroxyapatite is a calcium phosphate ceramic that is chemically similarto the mineral component of bone and will support bone growth.
Coatings of hydroxyapatite are often applied to titanium orstainless steel implants to improve surface properties.
SupraB is a polymer based on2-ureido-4[1H]- pyrimidinones.
EOS will beshowing an implant filled with the compound at MD&M. It was designed by ateam at AZM (the University Hospital Maastricht, The Netherlands) headed by Dr.Jules Poukens. A patent for the network of holes in the unique mesh scaffoldhas been submitted.
Lenz said hewill disclose the first validated in vivo (animal) results for laser-sinteredPEEK at an MD&M conference session. Human trials are planned for thefuture.
Lenz serveson a number of international standardization organizations, including ASTMCommittee F42 on additive manufacturing technologies.
The purposeof the EU program is to develop fully customized implants that are deliverablewithin two days and designed solely on the clinical needs of the patient usingenhanced rapid manufacturing technologies. A focal point is development ofadvanced biomaterials.
Selectivelaser sintering refers to an additive manufacturing technology in which ahigh-powered laser driven by a CAD file fuses tiny particles of metal orplastic in incremental layers.
About the Author(s)
You May Also Like






