The Challenges of Zinc-Air Batteries
Despite some attractive refueling arguments, rechargeable zinc-air batteries are still not yet ready for prime time.
February 28, 2023
Zinc-air batteries have been around for a long time. They are commonly used as a primary (non-rechargeable) battery in coin and button configurations in watches and hearing aids. By using air to provide oxygen as one of the reactants, zinc-air batteries can be made very light. Because zinc is abundant and cheap, the material costs of such batteries can be quite low. The problem comes when trying to make zinc-air batteries that are rechargeable.
In a primary zinc-air battery, zinc particles are incorporated into a porous anode paste that is saturated with an electrolyte. During discharge, at the cathode, oxygen from the air forms hydroxyl (OH-) ions that migrate to the zinc to form a zinc compound called zincate (Zn(OH)42-). During the formation of the compound, electrons are given up that are collected at the anode and pass through an external circuit, powering an electrical device and returning to the cathode. Meanwhile, the zincate is converted to zinc oxide and water, which returns to the aqueous electrolyte.
Reversing this process to recharge the battery is where difficulties arise. First of all, like lithium and many other metals, zinc suffers from unequal deposition on the anode side during charging. It forms spikey dendrite crystals that can grow large enough to short out the battery cell. A company called EnZinc has developed a sponge-like anode, using technology developed by the U.S. Naval Research Laboratory, that it claims helps reduce dendrite growth. At the cathode, electrically reversing the reaction to liberate oxygen from the hydroxyl ions and water is difficult. It requires catalysts that are often made from expensive metals like platinum, although research is underway to use more commonly available elements.
There is another way, however, around zinc air's limitations: mechanical recharging.
Instead of breaking apart, the zinc oxide discharge product at the anode, the entire anode and electrolyte can be replaced. Fresh zinc powder can be used so that the battery acts as though it was a new primary cell. The zinc powder and electrolyte can be combined into the consistency of a slurry, allowing it to be pumped into the battery. Meanwhile, the zinc oxide can be removed and converted back into zinc metal at an alternate processing site. This avoids the issues with dendrite formation at the anode.
Mechanical recharging of zinc-air batteries has been suggested as a way to rapidly recharge electric vehicles. This was demonstrated in electric buses in Singapore in the 1990s. A Canadian company called MGX Minerals developed a zinc-air flow battery aimed at electrical power grid support, replacing and augmenting diesel generators and marine engines.
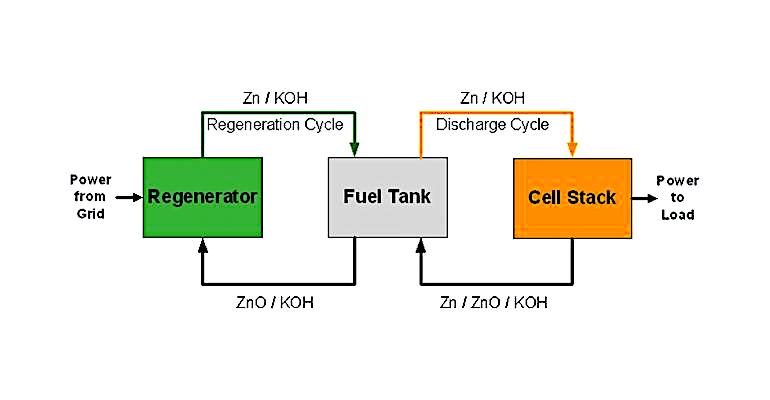
The power from the electrical grid operates a regenerator that converts zinc oxide into zinc and oxygen. The zinc is combined with a potassium hydroxide electrolyte, while the oxygen is released into the air. It is a continuous process, as long as power is applied to the regenerator. The metallic zinc particles and electrolyte are pumped to a storage tank, where they remain until power is needed from the battery.
To produce electricity, metallic zinc particles are drawn from the storage tank into a “cell stack” made up of individual battery cells. There, oxygen from the atmosphere is combined with the zinc metal, creating zinc oxide and releasing electrons. The zinc oxide returns to the regenerator, where it starts the process over again. MGX Minerals claims the process is easily scalable by increasing the size of the storage tank. Unlike a traditional battery, the system can also be charged and discharged at the same time. Notably, the cost of zinc is much lower than that of lithium.
Mechanical recharging is attractive, as it can provide the kind of “fuel tank” refilling, as with a gasoline-powered vehicle. By removing the “spent” zinc oxide and replacing it with fresh metallic zinc, it is possible to “recharge” the battery in minutes, rather than the hours that normal electrical battery recharging can take. The capacity—and thus the range for a vehicle with a mechanical recharging zinc-air battery—is dependent only upon the size of the zinc “fuel tank.”
A limitation in zinc air batteries is the electrical potential of 1.35-1.4 volts in practical cells—about half of what is available in a lithium-based battery. Another challenge is setting up the infrastructure for stations that could supply fresh metallic zinc in a slurry while removing and storing or renewing the spent zinc oxide. This is not insurmountable. But for now, it is easier to supply electricity from the grid through a charging station to a lithium-ion battery—the technology that seems destined to dominate electric vehicles, at least in the short term.
Kevin Clemens has been writing about energy, automotive, and transportation topics for more than 30 years. He has masters degrees in Materials Engineering and Environmental Education and a doctorate degree in Mechanical Engineering, specializing in aerodynamics. He has set several world land speed records on electric motorcycles that he built in his workshop.
About the Author(s)
You May Also Like



