Novel Electrification Method Used to Desalinate Water at Scale
Researchers modified an electrodialysis method for efficiency and cost-savings to provide a broadly accessible way to create drinking water through desalination.
July 7, 2023

Even in our modern world with all of its technological advances, billions of people are without easy access to fresh water. But scientists are trying to change that in various ways that can turn the salt water that covers two-thirds of the Earth's surface into drinking water. As part of this endeavor, researchers at the University of Illinois Urbana-Champaign have developed a new water desalination and purification system that takes a page out of the medical playbook. The solution—which removes salt and other unnecessary particles to create potable water—uses an electrified version of dialysis as the key part of its process. However, it modifies the typical way this process is used in key ways to create a more efficient and cost-effective solution, the researchers said.
The leader of the research—Xiao Su, assistant professor of chemical and biomolecular engineering at the university—said the team's aim was to find a low-energy and inexpensive way to purify water that can be useful for the underserved communities that need it the most.
“I see our solution as a platform to tackle both the energy and water crises,” said Su, also a researcher in the university's Beckman Institute for Advanced Science and Technology.
The researchers already have successfully applied their solution to wastewater with planned expansion into rivers and seas, they said. Moreover, it can save communities money and uses 90 percent less energy than comparable solutions, they said.
Altering the Desalination Method
The process of desalinating water is not as straightforward as it may seem and typically is expensive and uses a significant amount of energy, the researchers said. It's also complicated by the need to remove impurities and other organic matter that's also present in saltwater, which can drive up the cost even further.
Typical solutions used today require filtration or evaporation to separate the salt and other various materials in the water, often using heat. In fact, merely boiling salted water causes the liquid to evaporate and the salt to remain as a crust.
Knowing this, Su and his team took a different approach, one that's similar to blood dialysis used in medical scenarios but with a twist. They performed electrodialysis, which is like dialysis of the blood in that it flushes salt and other organic matter from wastewater in a similar way that dialysis uses the kidney to flush salt and other toxins from human veins, the researchers said.
One drawback to electrodialysis, however, is that it comes at a high-energy cost mainly due to its water-splitting reaction, they said. This pulls water molecules apart into two components: a positively charged proton and a negatively charged hydroxide.
Building blocks of salt have charges of their own, and so splitting the water forces the mineral’s movement in a designated direction, similar to the reaction between metal and a magnet, the researchers said.
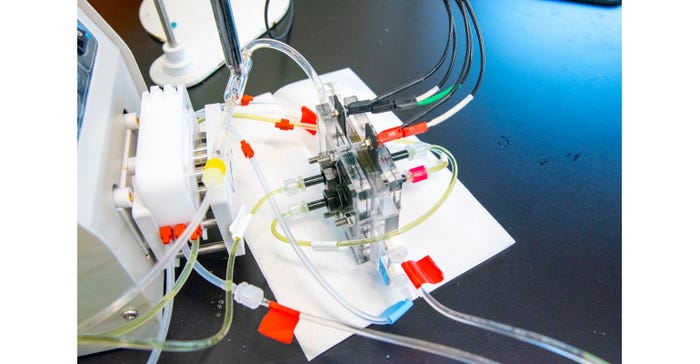
Modifying the Electrodialysis Process
Electrodialysis uses charged ion-exchange membranes—which are so-named because they allow only ions to pass through—for this reaction instead of a magnet, however. These membranes represent one of the most expensive components of electrodialysis, as they require constant upkeep and frequent replacement, the researchers said.
To avoid this costly part of the process, the researchers modified a traditional electrodialysis approach to water purification in two ways to save both energy and cost, they said.
One is that they streamlined the salt-separation process with a chemical phenomenon called a redox reaction, a combination of a reduction and oxidation process also known as a reaction used in large-scale energy-storage for renewable energies. To trigger this redox reaction, the researchers added a special polymer-based material to the wastewater before it is filtered and purified.
The results of this modification change the charge of the entire water molecule in one process rather than splitting it, achieving the same degree of separation while requiring about 90 percent less energy than traditional water-splitting, the researchers found.
To cut costs in addition to adding energy efficiency, the researchers also traded conventional ion-exchange membranes for nanofiltration membrane, a more robust and less expensive option, they said.
Potential for Desalination
The researchers published a paper on their work in the journal, ACS Energy Letters. They already have experimented with their solution at a regional water treatment plant, where it showed success at water purification, they reported. Future plans include expanding their experiments using saltwater and brackish water sources like groundwater and rivers.
Moreover, the researchers believe they can pair their solution with solar panels because of its low-energy requirement. This can lead to useful applications for both providing energy and purifying water in climate-affected regions, “where low-cost, low-energy desalination is very much needed,” Su said.
“Water scarcity is a global problem, and it’s not going to change in a day," he said. "But we are taking a step toward a solution that is feasible and capable of being scaled up."
And while so far the researchers have tested the solution only on multiple liters of water at a time, they hope to expand it so it can scale to much bigger water sources, Su said.
“The science is there, so the next step is paving a way for deploying these devices for real-world water treatment," he said. "I believe the time is right for that, and I’m excited to see it happen.”
About the Author(s)
You May Also Like



