Paper-Like Battery Electrode Aims at Space Exploration, Aero
A novel paper-like battery electrode made out of silicon oxycarbide glass and graphene could meet the specific needs of aerospace applications, such as those used in space exploration or unmanned aerial vehicles
May 12, 2016
Aerospace applications often have unique demands for batteries that power devices and vehicles, with special requirements to operate at very high or very low temperatures, as well as other features.
Researchers at Kansas State University recently developed a novel paper-like battery electrode out of silicon oxycarbide glass and graphene that could fit the bill to meet the specific needs of aerospace applications, such as those used in space exploration or unmanned aerial vehicles (UAVs).
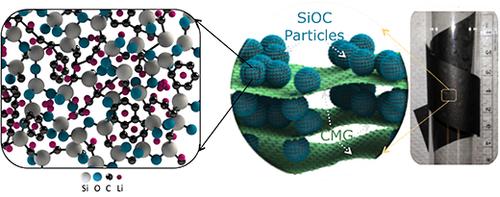
Gurpreet Singh, an associate professor of mechanical and nuclear engineering, and his research team created the battery electrode using silicon oxycarbide-glass and graphene, a combination of materials that until now has been difficult to incorporate into practical batteries, they said. This is because of challenges that arise at high mass loadings, such as low capacity per volume, poor cycling efficiency, and chemical-mechanical instability, according to researchers.
To address these challenges, Singh and his team placed a glass-like ceramic called silicon oxycarbide between large platelets of chemically modified graphene (CMG), he said. The resulting electrode has a high capacity of approximately 600 miliampere-hours per gram, or 400 miliampere-hours per cubic centimeter, thanks to the silicon oxycarbide, which in and of itself is a unique material, Singh said.
Gurpreet Singh, associate professor of mechanical and nuclear engineering at Kansas State University, and his research team have developed a paperlike battery electrode using silicon oxycarbide glass and graphene, a material that has benefits over typical materials used in batteries today, he said.
(Source: Kansas State University)
To prepare silicon oxycarbine, liquid resin is heated to the point where it decomposes and transforms into sharp glasslike particles. This causes the silicon, carbon, and oxygen atoms to rearrange into random 3D structure, with any excess carbon precipitating out into cellular regions, he explained.
The open 3D structure creates large sites for reversible lithium storage and smooth channels for lithium-ion transportation that is different than the typical crystalline silicon electrodes, according to Singh. The material also allows for low-cost electrodes to be fabricated, because liquid resin is a widely available byproduct of the silicone industry.
The team achieved the paperlike design of the electrode—also different from other electrodes in that it eliminates the metal foil support and polymeric glue—from 20 percent chemically modified graphene platelets, Singh said. This allows for a greater capacity ability because those additional materials do not contribute toward the this aspect of the battery, he said.
The resulting design also is lightweight, saving 10 percent in total weight, and has the capability to store lithium-ion and electrons with near 100 percent cycling efficiency for more than 1000 charge discharge cycles, according to the team. The material also is able to demonstrate its performance at practical levels even in low temperatures, which makes it suitable for UAVs or applications in space at high altitudes, Singh said. The paper electrode cells in tests delivered a capacity of 200 miliampere-hour per gram even when kept at minus 15 degrees C for about a month, which he noted is remarkable compared to other batteries.
READ MORE ARTICLES ON BATTERY RESEARCH:
Singh and his team published the findings of their research in an article in the journal Nature Communications. The team plans to move forward and address the practical challenges of the electrode, hoping to be able to produce the material at even larger dimensions for a wider range of applications, he said.
"Ultimately, we would like to work with industry to explore production of lithium-ion battery full-cells," Singh said. "Silicon oxycarbide can also be prepared by 3D printing, which is another area of interest to us."
Elizabeth Montalbano is a freelance writer who has written about technology and culture for more than 15 years. She has lived and worked as a professional journalist in Phoenix, San Francisco, and New York. In her free time she enjoys surfing, traveling, music, yoga and cooking. She currently resides in a village on the southwest coast of Portugal.
About the Author(s)
You May Also Like



