Want Speed in an Emergency? FDA Praises 3D Printing Response During COVID
The FDA has released a report on the value of additive manufacturing during the pandemic.
July 19, 2021
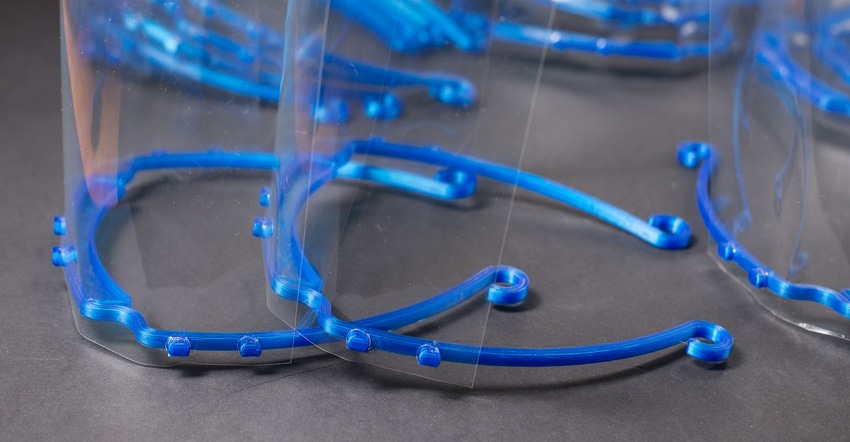
Apparently, the role of 3D printing in solving equipment issues during the pandemic was more extensive than most realized. In a new report – Executive Summary: Assessing the Role of Additive Manufacturing in Support of the U.S. COVID-19 Response -- the FDA notes that starting in March 2020, the US healthcare community experienced unprecedented shortages of personal protective equipment (PPE), PPE accessories, and medical devices to treat COVID-19 patients.
American individuals and companies responded to the needs of the healthcare community by designing and producing additively manufactured PPE, PPE accessories, and medical devices. Those responding included hobbyists, communities, and small and large companies that leveraged the adaptability of additive manufacturing (AM).
3D Printing Companies Rushed in to Help
Protolabs was one of the many 3D printing companies that dropped everything to produce needed medical equipment. Company leaders realized a rapid response to medical equipment shortages was paramount. “That speed was critical during the pandemic. We have always prided ourselves on speed but that accelerated during the pandemic,” Gurvinder Singh is the global product director for injection molding at Protolabs, told Design News. “We were shipping parts on Monday after getting the order on Friday.”
Protolabs revamped its production for medical equipment manufacturing at the very beginning of the pandemic. “Late February and early March was the start of the demand. We transitioned into an end-user supplier. Our customers pushed us into that,” said Singh. “We saw the need for the low volume high mix. Yet we were manufacturing this at high volume,” said Singh. “We started seeing the need for ventilator parts. Then testing kits, then PPE, masks, and face shields.”

The Pressing Needs During COVID
The healthcare community faced critical shortages of PPE, PPE accessories, and medical devices necessary to manage COVID-19 transmission due to several factors:
1. Reliance on just-in-time manufacturing
2. Lack of an adequate and well-maintained national PPE stockpile
3. Reliance on globally sourced products from countries that were simultaneously being hit with the same pandemic
Failure to acquire adequate PPE, PPE accessories, and medical devices was about to jeopardize the stability of the entire healthcare system and its ability to provide quality care to Americans suffering any number of ailments. Such shortages affected not only major hospitals but also long-term care and nursing facilities, ambulatory health services, and social services. The medical community faced shortages of N95 respirators, face shields, gowns, COVID-19 diagnostic nasal swabs, ventilators, and more.
Rationing Equipment Was Not Enough
The Centers for Disease Control (CDC) tried to alleviate the problem by suggesting rationing the equipment. The CDC directed healthcare providers to practice crisis capacity N95 respirator stockpile management and reuse practices. However, other items the medical community lacked provided the opportunity for AM to address traditional supply shortages.
PPE accessories and medical device items produced by AM technology included a handful of key pieces of equipment:
1. Face shields were one of the most produced pieces of additively manufactured PPE, and they were easily used by all members of the medical community.
2. Ear savers and other non-medical devices were both easy to AM and readily used by healthcare professionals because they were not replacing designated medical devices.
3. The development of AM printable nasal swabs, particularly nasopharyngeal swabs, enabled hospitals to mitigate shortages of diagnostic equipment for COVID-19.
4. Face masks were a strong initial focus of the medical community, but due to their criticality in infection control, the healthcare community focused on N95 stockpile management and N95 reuse strategies. pivoted operations to additively produce PPE, PPE accessories, and medical devices.
Equipment Producers Came from Everywhere
Community producers included individuals, academia, hospitals, makerspaces, and government agencies that used AM machines to create PPE, PPE accessories, and medical devices. These producers relied on prior manufacturing experience, knowledge of AM techniques, in addition to the National Institutes of Health 3D Print Exchange and the COVID 3D TRUST, to quickly design and produce safe items. The rapid response hinged on collaboration through social media, personal connections, and professional organizations. This resulted in numerous local responses across the country.
Rob Spiegel has covered manufacturing for 19 years, 17 of them for Design News. Other topics he has covered include automation, supply chain technology, alternative energy, and cybersecurity. For 10 years, he was the owner and publisher of the food magazine Chile Pepper.
About the Author(s)
You May Also Like





