As we learned at BIOMEDevice Boston, newer soft tissue implants must be lighter in weight and manufactured with less overall material. The same goes for medical packaging.
April 20, 2016
Lots of medical implants for body structures will continue to be made of strong stuff like metals and tough plastics. Some plastics are even being developed to meet the needs of different types of implants, with tunable characteristics such as antimicrobial properties. But there's also a trend away from passive, permanent implants to absorbable ones that aid wound healing and then dissolve when their job is done.
Those soft tissue implants must be lighter in weight and they must be manufactured with less overall material, Rick Crane, vice president of the innovation services group for J-Pac Medical, told Design News at UBM's BIOMEDevice conference last week in Boston. Among other things -- forming, assembly, and packaging for a broad range of materials -- the company manufactures anatomically correct, class III implantable textile assemblies.

The lightweight materials used to manufacture soft tissue implants, including absorbable ones and those used for drug delivery, are textiles, usually made of polyester and polypropylene.
The materials used to manufacture these implants are in the form of textiles, said Crane. These textiles are usually made of polyester and polypropylene, which have been used for years in implants destined for soft tissue. J-Pac Medical, which does contract manufacturing and product development for implants and medical devices, had examples of these materials on display at its booth. Many of them look like netting or fine screens. Some of the implants made with them are absorbable, and some may be used for drug delivery.
For biomedical, implantable textiles and films, the company provides forming, cutting, and assembly, and is experienced in creating both two-dimensional and three-dimensional structures that are custom-shaped to meet customers' biological and anatomical requirements. "Some engineers on the medical device side are starting to look at these textiles, and figuring out how to use them in devices," said Crane. "There may not be any applications for them today, but there will be tomorrow." Those applications are likely to be for devices that aid soft tissue repair in orthopedics, general surgery, and cardiovascular uses.

Some engineers designing medical devices are starting to look at these textiles and figuring out how to use them for future applications that aid soft tissue repair in orthopedics, general surgery, and cardiovascular uses.
On the medical device side, J-Pac Medical uses tooling and automation to manufacture customers' finished sterile devices, including high-precision assembly. It also consults with customers on design-for-manufacturability issues during product development, said Crane.
The company also does medical packaging, such as sterile disposable packaging for implants, devices, and surgical instruments, and recently announced new dry room processing capabilities for packaging environmentally sensitive healthcare products. In general, medical packaging is also downgauging in an attempt to use as little material as possible, said Crane. Light, strong, and rigid are the key characteristics often needed for thermoformed trays, with some customers needing more flexible packaging. The main materials here are PETG (polyethylene terephthalate glycol-modified) and polystyrene trays for EtO (ethylene oxide) and gamma sterilization processes, plus polycarbonate, which can also be used for these two, as well as for autoclave sterilization.
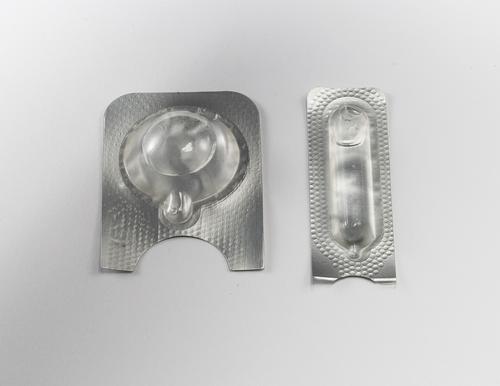
A new trend in medical packaging is blister packaging for containing lab-on-chip diagnostic reagents, used in point-of-care testing. These are quite small, about the size of some blister packs for over-the-counter pills.
A new trend in packaging J-Pac Medical sees is the use of blister packaging for containing lab-on-chip diagnostic reagents. "This is very new, you could say embryonic," said Crane. "We're still doing a lot of tests." These blister packages are being made in customized formats for point-of-care testing. The company also had examples of these on exhibit, and they're quite small, about the size of some blister packs for over-the-counter pills.
[images via J-Pac Medical]READ MORE ARTICLES ON MEDICAL IMPLANT MATERIALS:
Ann R. Thryft is senior technical editor, materials & assembly, for Design News. She's been writing about manufacturing- and electronics-related technologies for 28 years, covering manufacturing materials & processes, alternative energy, and robotics. In the past, she's also written about machine vision and all kinds of communications.
About the Author(s)
You May Also Like



