COVID-19 has prompted many manufacturers of medical devices, point-of-care testing and near-patient testing products, and medical wearables to reassess and upgrade their assembly fluid dispensing capabilities—from manual applications to benchtop dispensing, to lab testing and in-line automated production.

The medical device and life sciences industry must meet stringent regulations for quality and product consistency, making process control a critical issue. All materials and manufacturing processes—including machining, assembly, and packaging—must be documented for complete traceability and process validation. This is critically important with fluid dispensing applications in the assembly of medical devices, point-of-care testing (POCT) and near-patient testing (NPT) products, and other life sciences applications such as medical wearables. These applications require accurate and consistent deposition of fluid amounts of UV-cure adhesives, cyanoacrylates, silicones, and other fluids in their manufacture.
Regardless of the application, the fluids being dispensed, and the dispensing technique, the dispensing method must also meet requirements for volume throughput and cost efficiency in addition to maintaining quality. Any increase in production volume requirements when relating to assembly is often the key driver that necessitates the move to a more-efficient dispensing system.
Responding to COVID-19
This is particularly true right now as medical product manufacturers worldwide have been increasing production of critical supplies and medical devices to address COVID-19 (SARS-CoV-2). Dedicated to helping manufacturers increase production capacity to meet this growing demand, fluid dispensing equipment manufacturers have come forward with precise and safe dispensing solutions to mass produce ventilators, diagnostic test kits, and other medical devices.
Responding to requests early on in the COVID-19 pandemic, automated robotic fluid dispensing systems were provided to mass produce a small sub-assembly inside Ventec Life Systems’s unique ventilators. Ventec’s critical care ventilators are portable and have provided front-line medical professionals with the systems they need to fight COVID-19. The sub-assembly application required bonding two components together using a UV-cure acrylic.
Prior to the pandemic, Ventec was using manual dispensing techniques. COVID-19 forced the company to quickly expand to meet a 180 percent increase in production volume per month. This was achieved, in part, by utilizing the automated robotic fluid dispensing systems.
Another high-priority COVID-19-related application involved jetting medical reagents onto diagnostic test strips and bonding the housing of test cards for COVID-19 test kits. PICO Pulse jetting systems were provided for these applications, owing to their fast dispensing speed and extremely high precision.
These applications are characteristic of manufacturers that needed to scale the sophistication of their fluid-dispensing processes to meet COVID-19 increased-production requirements. In most high-volume manufacturing environments, automated and semi-automated fluid dispensing applications may be in use, dependent on the throughput volume and quality standards required at any stage in the assembly process.
Scaling Dispensing Automation to Meet Production Requirements
Many medical device manufacturers likely start out with manual squeeze bottles and medical syringe dispensing. Then, as production volumes increase, some progress into employing more controlled approaches with precision benchtop fluid dispensers, pneumatic valve systems, or in-line robotic dispensing systems, for at least part of their fluid dispensing.
There are a number of factors that would support adopting a more-efficient and controlled dispensing method as a better business solution:
Shot-to-shot repeatability and accuracy are considerably improved as a more automated and controlled dispensing approach is employed.
Increased productivity is clearly a benefit that comes with increased automation. For example, the same worker who manually assembles 800 parts during an eight-hour shift can assemble 1,000 to 1,200 parts with the assistance of a pneumatic fluid dispenser.
Part quality improves when switching from manual squeeze bottle dispensing to air-powered dispensing and further along to in-line automated dispensing, because operator-to-operator variance is significantly reduced. The ability to set the time, pressure, and other dispensing parameters for an application improves process control and ensures the right amount of fluid is placed on each part.
Rework and reject rates lessen when upgrading to more-automated dispensing solutions, thus improving the yield of the manufacturing lines and greater profitability to the manufacturer.
The amount of assembly fluid used decreases significantly when using a more-controlled method of dispensing. Switching from a rudimentary manual dispensing process, for example, to a pneumatic dispenser can cut the amount of fluid used typically from 50 to 70 percent given the improved accuracy of the deposit.
Medical device manufacturers can greatly benefit by taking a closer look at their production requirements and embracing a more-controlled and automated fluid dispensing capability. It is critical, however, to consider each of the five points above, as they represent the actual cost-to-benefit factors influencing fluid dispensing processes.
Repeatability, Traceability, and Process Control
Transforming fluid dispensing from a more-manual procedure to a more-automated process not only provides cost savings from labor and fluid waste, but it more importantly delivers a higher level of consistency, reliability, and traceability in the fluid dispensing process. Such consistency, reliability, and traceability are of the utmost importance in medical device assembly.
Repeatability
Shot-to-shot repeatability and accuracy are critical factors in fluid dispensing and with particular importance in the manufacture of medical devices. Depositing the right amount of fluid has a compounding consequence of keeping downstream production moving. If too much fluid is applied, the longer it can take to cure, which will delay production downstream. Conversely, if too little fluid is applied, the part will not properly bond, again interrupting downstream assembly or causing a failure in the product. Precision dispensing systems apply shot-by-shot repeatable amounts of virtually any manufacturing fluid, by using digital timers and precision air regulators to determine the amount of material applied.
The latest generation of fluid dispensers can distribute practically all assembly fluids—from thin solvents to thick silicones and brazing pastes—with greater accuracy. They deliver exceptional throughput and process control, with consistent deposits from the beginning to the end of the fluid reservoir.
For the precise application of adhesives, lubricants, paints, solder pastes, two-part epoxies, UV-cure adhesives, and other assembly fluids, precision dispensing systems enable optimal results.
The consistency and repeatability performance of precision dispensing systems goes beyond the actual dispensing equipment itself and is also dependent upon the quality and proper usage of the system components. These consumable plastic components—syringe barrels, adapter assemblies, pistons, caps, and dispense tips—are designed to meet the requirements of different types of fluids and applications and to dispense the most precise fluid deposit possible.
To achieve the highest level of performance from these dispensing systems, several requirements need to be inherent in their manufacture and usage:
Each of the consumable plastic components should be designed as part of a complete, integrated system. This will improve yields and reduce costs by producing the most accurate, repeatable fluid deposits possible. Mixing and matching components from different systems or suppliers is a recipe for diminishing performance.
The dispensing components should always be used as single-use consumables. In high-precision dispensing systems, barrel IDs (internal diameters) and piston diameters, as well as dispensing tips, are manufactured with tolerances that make any residue from prior dispensing residing in the barrel, piston, or tip degrade dispensing repeatability performance. Once the piston reaches the bottom of the barrel, the barrel, piston, and tip should be discarded.
Maintaining precision and shot-to-shot repeatability in dispensing starts with quality manufacturing of the components. For best performance, all components should be certified that no silicone mold-release agents are used in the precision molding process or at any other time during the production of the dispensing components.
Some dispensing solution providers also provide a complete dispensing component system molded from medical-grade United States Pharmacopeia (USP) Class VI resin. These syringe barrels, pistons, and end and tip caps are designed to simplify process validation for medical manufacturers. These and even standard dispensing components can be sterilized prior to use in medical device manufacturing.

Traceability
Most medical device components have a unique bar code assigned to them as they move through the production/assembly process to facilitate keeping track of the components throughout production—a system often utilized by industries other than medical device manufacturing for six sigma process controls.
New to the world of fluid dispensing is the unique capability to switch between stored dispensing programs using nothing more than a bar code scanner. This allows the operator to change the parameters for a new application without touching process parameters on the touchscreen of this new benchtop fluid dispenser. The dispenser settings automatically switch when the new program bar code is scanned.
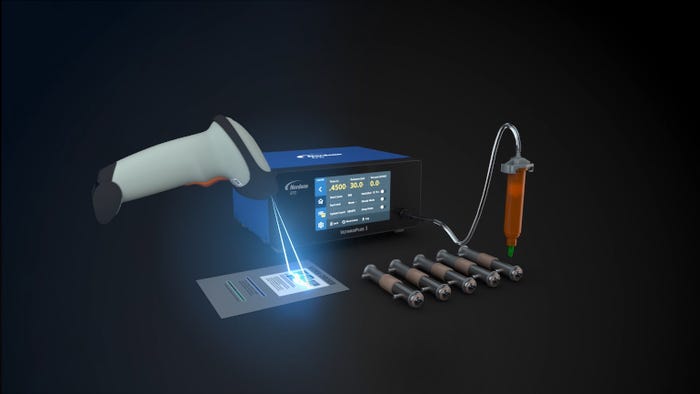
The days of maintaining dispensing parameters in handwritten spreadsheets and notepads and manually entering coordinates into the dispenser on subsequent runs are now in the past, greatly reducing the possibility of human error in setting dispensing parameters.
Another unique feature is a digital dispense log, which automatically records data on dispense parameters—such as dispense time, pressure, vacuum, and the date, day, and time of each dispense cycle. Manually downloadable through the dispenser’s USB port, the dispense log is beneficial to manufacturing processes that require stringent, documented process control, especially in life sciences applications to meet FDA or EU MDR (Medical Device Regulation) requirements, or that of other global medical device regulatory bodies.
Process Control
The ability to set the time, pressure, and other dispensing parameters for an application improves process control and ensures the right amount of fluid is placed on each part.
The latest generation of benchtop dispensers provides a high degree of process control for dispensing applications in the assembly of medical devices, capable of dispensing adhesives, solder pastes, and all other assembly fluids with high consistency.
Fluid dispensing of dots, beads, and fills can be achieved with dispensing equipment features such as a 1-100 psi air pressure regulator, timed-shots, vacuum control to keep thin fluids from dripping, digital time/pressure displays, and electric foot pedals.
Additional features include:
a) time adjustment as fine as 0.0001 seconds.
b) constant-bleed air pressure regulation (offered with Ultimus I and II dispensers) for reliable control when dispensing any type of fluid.
Some of the latest fluid dispensers allow programmable sequencing to automatically adjust dispensing parameters, making them ideal for applications that involve two-part epoxies and other fluids that thicken over time or get thinner as ambient temperatures rise.
Another feature supporting precision dispensing, which is particularly applicable for medical device manufacturers, is automated optical inspection (AOI). When coupled with CCD cameras and confocal lasers, vision guided automation platforms provide optical assurance of fluid deposit volume and placement accuracy ensuring a conforming deposit.
Using the robot's existing vision systems, the AOI software verifies fluid deposit widths and diameters. With the AOI confocal laser, the system measures the height of a fluid deposit in addition to the width and diameter, providing 3D deposit verification and determining whether dispense requirements have been met. The confocal laser detects deposit height measurements regardless of the transparency of the fluid, which can sometimes distort quality data. Constant closed-loop feedback delivers automated quality control data, saving medical manufacturers time and costs.
Benchtop Fluid Dispensing
It is the nature of medical devices that often warrants a more personalized and manual fluid dispensing approach in their assembly, compared with employing a more automated, robotic system. Often, the geometry of the parts is too complex to make automation a viable option. Or the production volume is too low to warrant the investment.
Because benchtop fluid dispensing has proven to be a highly workable and reliable method for assembly of medical devices, fluid dispensing manufacturers have routinely improved and refined their systems. Pneumatic benchtop fluid dispensing has taken a considerable leap forward in both simplicity and ease-of-use, and improved process control, to further support the stringent requirements of medical device manufacturing.
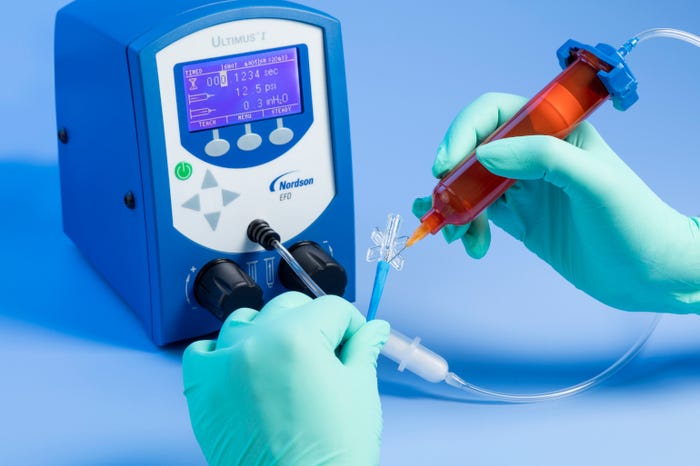
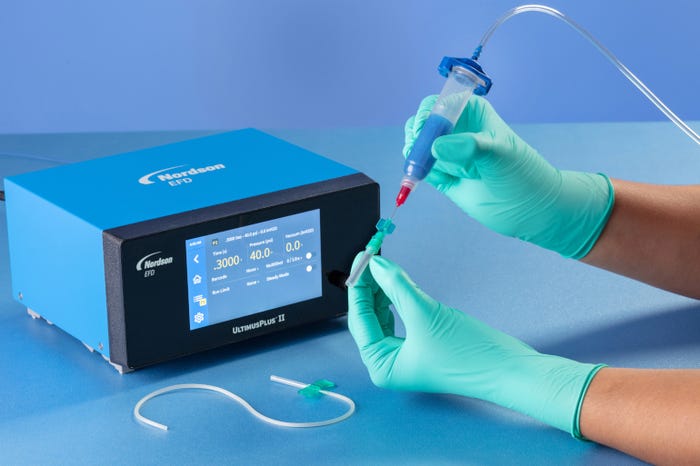
These improvements are designed to facilitate easier and more efficient operation of the fluid dispensing process from the operator’s perspective and maintain better process control over dispensing parameters to effect more consistent dispensing outcomes.
These latest changes also bring semi-automated desktop fluid dispensing into compatibility with Ethernet, the Industrial Internet of Things (IIoT), and the cloud, enabling the potential for more direct machine/process interconnectivity. Although this is particularly beneficial where benchtop fluid dispensing systems are used in combination with robotic dispensing applications, aspects of this enhanced connectivity can be beneficial, right now, to non-robotic pneumatic benchtop fluid dispensing. In essence, these new improvements enable the benchtop dispense technician to begin to benefit from smart-factory digitization.
Following is an overview of the unique capabilities integrated into these new benchtop fluid dispensers.
Automated Pressure Regulation with Full Operator Lock-Out
In the assembly of medical devices, it is critical that the fluid dispensing be administered precisely and in the same repeatable pattern on each component with repetitive standardizations.
Typically, fluid dispensers allow the operator to manually adjust the pressure regulation. On some of the latest benchtop fluid dispensers, the operator can now be fully locked out from manually adjusting the pressure regulation as well as other dispense parameters like time and vacuum settings. Without access to an assigned password, the operator cannot change the dispense program parameters, which provides the next level of process control at a lower cost of ownership than was previously possible.
This full electronic pressure regulation provides full operator lock-out. It alone enhances dispense quality control by eliminating process inconsistencies caused by operator-to-operator variability.
Facilitating Faster Set-Up and Integration
The newest benchtop fluid dispensers are equipped with a USB drive, which not only permits dispense reports to be output onto a flash drive but also enables distribution of dispense programs into and out of the dispenser. This can considerably cut programming time when multiple dispensers are set up to dispense the exact same parameters on the same parts. One dispenser can be set up with multiple programs, and then those parameters can be output to a flash drive and loaded into the other dispensers, minimizing human error and expediting loading of dispense programs across the factory floor.
Also speeding fluid dispensing set-up and operation for medical device manufacturers is HMI touchscreen control for input of dispensing parameters and management of all dispense process functions. This brings with it a new level of usability for operators and production managers.
Touchscreen control makes setup significantly faster than it was with analog knobs and push-button controls.
The dispense cycle can be initiated by a centralized, customer-site-specific PLC as part of a large, in-line operation. When the production line needs to move from making one type of part to another, the PLC will remotely trigger the dispenser to change the program and dispense a different deposit pattern.
In-Line Automated Robotic Dispensing
Automated robotic dispensing has evolved to support the needs for higher throughput medical device and medical diagnostics assembly production by developing specialized dispensing technology for automated inline assembly and manufacturing systems and for stand-alone production devices. In these demanding applications, small amounts of adhesives, silicone, and other fluids must be dispensed reliably and accurately in dosage, placement, and repeatability. The precise positioning and quantity of these fluids deposited is crucial to the products’ assembly, function, quality, appearance, and viability.

Highly automated, in-line jet dispensing technologies deliver very discrete, highly precise microdots of fluid onto substrates within extremely short cycle times, at rates of up to 1,000 cycles per second, and with infallible repeatability.

In most applications, a series of small micro-deposits are being put down dot-to-dot, which stitch together, blending seamlessly to make a continuous line or pattern. Any shape or solid can be created on a substrate by stitching dots with micro-dispensing. Multiple microdots can be stacked to form larger dots. Dispensed microdots are 300–400 microns in diameter. Within these parameters, real-world applications typically require many different size dot diameters to accommodate the different size components that populate a substrate.
Given the requirements, more advanced micro-dispensing technologies can deliver extremely tight deposit tolerances that are within 1 percent of project specification for size, height, and shape of the microdots.
There are two basic types of in-line fluid micro-dispensing techniques: classic needle-based contact dispensing and non-contact jet dispensing. Although traditional contact fluid dispensing is still the predominant technology in use, it is hampered by definite disadvantages of speed, particularly when dispensing on irregular substrates. While the best method is ultimately determined by the material and application at hand, for many automated fluid dispensing processes, high-speed jetting technology is a popular and innovative alternative to traditional, needle-based contact dispensing.
These high-speed, high-throughput solutions are the dispensing methods of choice for point-of-care testing and near-patient testing products, particularly when employing needle-bonding.
Requirements for in-line automated dispensing are as varied as the differences in production environments. Many questions need to be addressed, such as:
Is the micro-dispensing to be used for a manufacturing/assembly process?
If so, is it to be utilized within an industrial environment, or possibly within a cleanroom?
Or is the system to be used within a production process, possibly producing prototypes?
Are these processes semi-automated or full-automated?
How skilled are the operators?
And more specific questions would determine expected parts per hour to be produced, production cycle times, and speeds that the parts can be fed into placement for dispensing.
Identifying these requirements will facilitate the selection of the most optimum dispensing and robotics system for the application.

Assessing the Right Dispensing Process
Because so many factors can impact fluid dispensing for medical device and medical diagnostic products, it is important to consult an experienced fluid application specialist who knows the specifics and priorities influencing dispensing in these applications.
Lab testing, prior to installation, is a crucial step in the process of selecting the most applicable system. Consulting with an application specialist early in a project will ensure the right fluid dispensing system is utilized, and the most optimum process has been put into place. This will facilitate manufacturing to achieve the desired production throughput and improve process control, while reducing rework, rejects, and fluid waste.
About the Author(s)
You May Also Like



