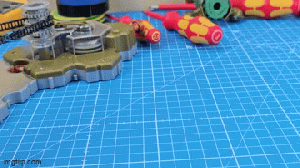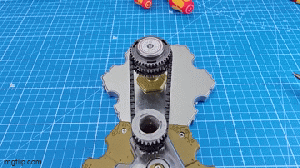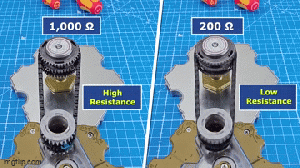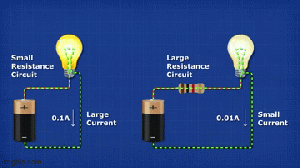
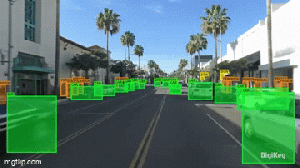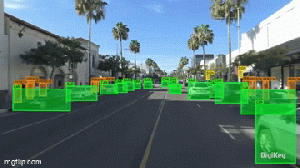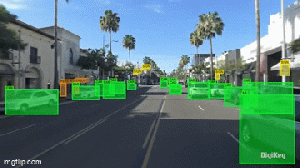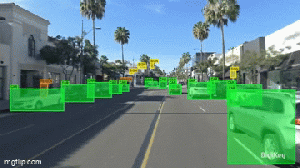
Vacuum tubes were instrumental in the development of early programmable computers.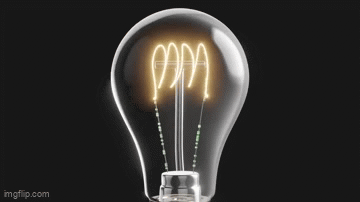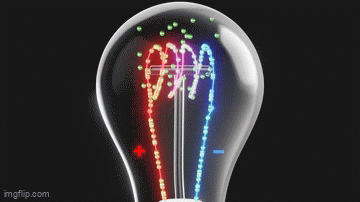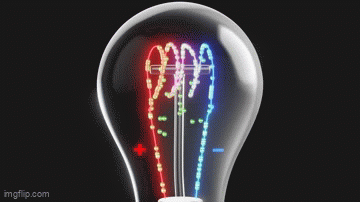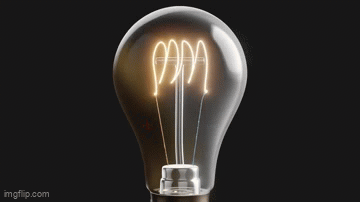
Electronics
What Do Light Bulbs and Vacuum Tubes Have to Do With Computers?What Do Light Bulbs and Vacuum Tubes Have to Do With Computers?
The humble beginnings of computers are rooted in early electronic devices that would eventually lead to the data-crunching machines we are familiar with today.
Sign up for the Design News Daily newsletter.






.jpg?width=300&auto=webp&quality=80&disable=upscale)