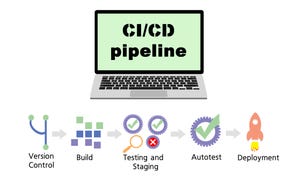
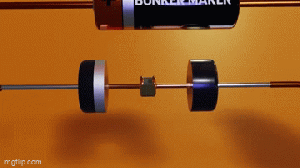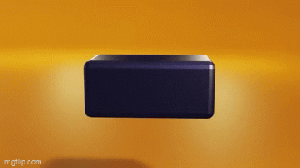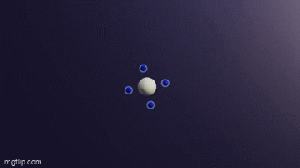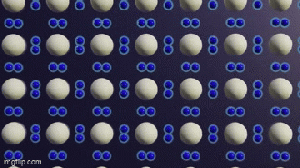
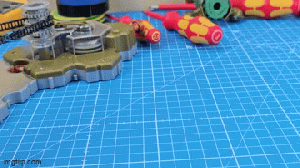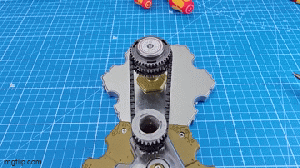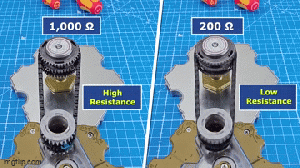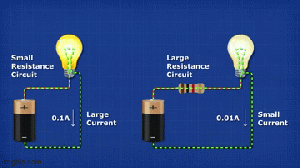
Wireless chargers involve tradeoffs. 
Batteries/Energy Storage
What You Should Know About Wireless ChargingWhat You Should Know About Wireless Charging
This video says the convenience of being able to charge your devices without wires comes with tradeoffs.
Sign up for the Design News Daily newsletter.










.jpg?width=300&auto=webp&quality=80&disable=upscale)