SMART Modular CXL Add In Cards ease memory expansion.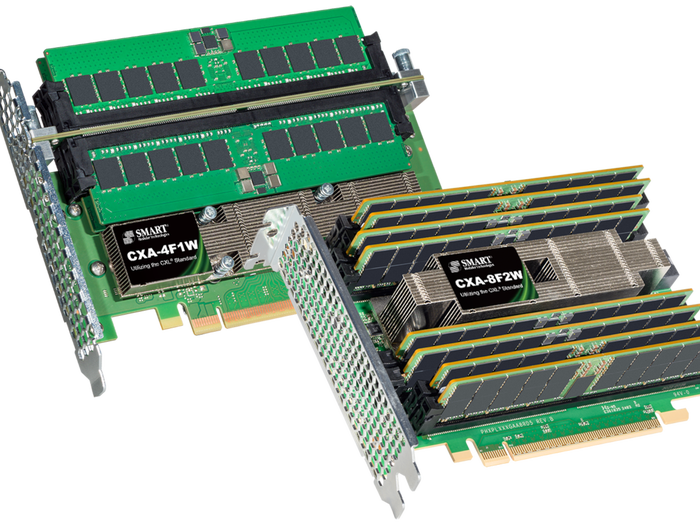
Embedded Systems
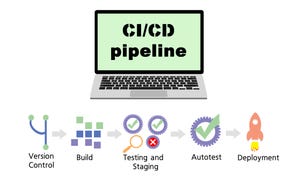
Add-in Cards Help Meet Expanding Server Memory NeedsAdd-in Cards Help Meet Expanding Server Memory Needs
CXL-compatible cards provide space- and cost-effective way to add server memory.
Sign up for the Design News Daily newsletter.















.jpg?width=300&auto=webp&quality=80&disable=upscale)