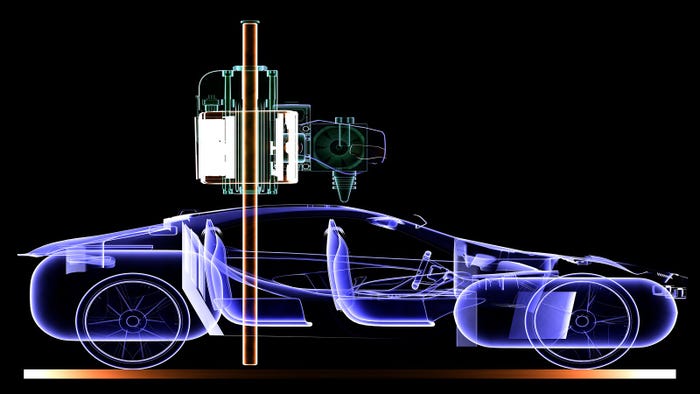
Top engineering grad programs
Industry
Top Engineering Grad Programs by PayTop Engineering Grad Programs by Pay
Nabbing a master’s degree can boost your salary. Take a look at the average pay for master’s graduates by school.
Sign up for the Design News Daily newsletter.